Creating, Researching, and Developing
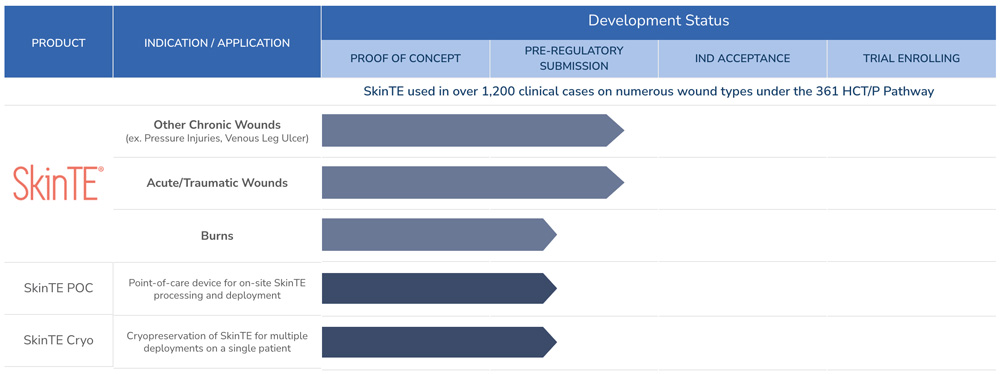
PolarityTE is focused on creating and developing products that address critical unmet needs within wound care. Our lead investigational product in development, SkinTE®, was previously marketed and used in over 1,200 clinical cases, often for patients who suffered from chronic cutaneous ulcers and had no other alternative form of treatment.
PolarityTE has an accepted investigational new drug (IND) application with the U.S. Food and Drug Administration (FDA) for the SkinTE® product, and has commenced its first pivotal clinical study in diabetic foot ulcers (DFU) to generate the data that the FDA requires for submission of a biologics license application (BLA).